Closing the gap between science & regulation to prevent unnecessary animal testing
No animal tests are carried out on finished detergent products, and A.I.S.E. is committed to preventing unnecessary testing of ingredients used for its products on animals. This will be achieved by closing the gap between science and regulation, i.e. finding alternative approaches of complying with EU legislation that focuses on the replacement, reduction, and refinement, a concept known as the "3Rs” of animal uses, through better and more predictive science. Such work is a collaborative effort between many stakeholders.
Ensuring compliance with REACH
The safety of detergent ingredients is regulated under
REACH. A.I.S.E. advocates for the proper use of New Approach Methodologies (NAMs) in the revision of REACH, which offer opportunities to prevent unnecessary animal testing. A.I.S.E. and its members are actively contributing to activities on NAMs through refinement of use and exposure for detergents ingredients and other alternative approaches to animal testing.
In addition, A.I.S.E. has a long history of pro-active product stewardship initiatives that cover among others the safe handling and use of enzymes in detergent products.
A number of regulatory drivers/challenges are facing the industry for compliance of detergent ingredients using non-animal approaches. In particular, there is no agreed mechanism or approach to address current hazard endpoint requirements using NAMs-based approaches. However, this presents potential opportunities for considering how modern NAMs-based science can be best applied to inform relevant safety decisions without using animal testing. These drivers include the following examples:
- Current and upcoming changes to REACH (hazard data under PBT/vPvB / PMT/vPvM and safety assessment)
- REACH surfactant category registrations (e.g. endpoint data requests vs. use of Weight of Evidence (WoE) and application of robust read-across approaches)
- Classification schemes :
- Globally Harmonised System of Classification and Labelling of Chemicals (GHS)
- EU Classification, Labelling and Packaging of Substances and Mixtures (CLP) Regulation (EC No 1272/2008) → new hazard classes introduced by Commission Delegated Regulation (EU) 2023/707
Engaging in scientific research through partnerships
A.I.S.E.’s commitment to finding alternatives to animal testing is implemented through our scientific engagement in a variety of research initiatives.
One such partnership for advancing scientific research is the joint platform of industry stakeholders and the European Commission known as the European Partnership for Alternative Approaches to Animal Testing, or
EPAA. Together, all the stakeholders are striving for scientific integrity, transparency, and construction collaboration. A.I.S.E. has been a partner of EPAA since its establishment in 2005.
Currently, A.I.S.E. members are heavily involved in the EPAA project "Non-animal science in regulatory decisions for chemical safety” with the following focus:
- Future classification system for human Systemic Toxicity: In 2023, during a workshop entitled ‘Towards an animal free regulatory system for industrial chemicals’ which took place at the European Chemicals Agency (ECHA), EPAA launched its designathon challenge for human systemic toxicity. The work involves searching for prototype NAM-based solutions to inform the development of a potential future classification system for human systemic toxicity. Read more
ERASM is a scientific research partnership between the detergents (A.I.S.E.) and the surfactants industry (CESIO) that has carried out joint scientific research on surfactants and their impact on the environment and human health for over three decades.
Some of the challenges and opportunities in relation to NAMs for surfactants include:
- Weight of Evidence (WoE) approaches should be encouraged to maximise available data from all non-animal sources;
- Chemistry issues: ionisables/surfactants are often outside current proven applicability domain of existing assays (e.g. OECD 249, OECD 319). The amphiphilic nature of surfactants can also make the determination of key properties challenging (e.g. water solubility, octanol-water partition coefficient (KOW))
- Additional robust alternative options are needed that cover the relevant chemical space (e.g. bioaccumulation and chronic fish toxicity prediction and identification of potential endocrine active substances (EAS)).
- Additional robust models to better understand or determine ‘internal’ concentrations f chemicals in relevant organisms at target site (e.g. Physiologically Based Kinetic (PBK) models) are needed to facilitate application / uptake of new in vitro methods.
- Challenge of Endocrine Disruption (under CLP) requires new thinking to adequately and reliably assess potential EAS using non-animal approaches.
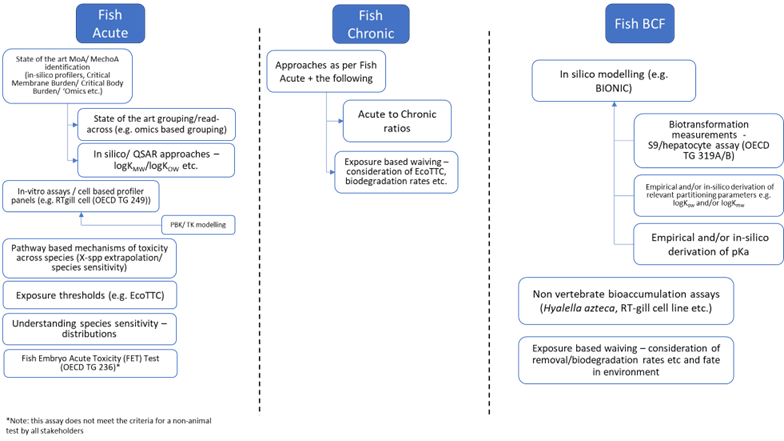
Figure 1: Schematic framework showing Weight of Evidence (WoE) opportunities using current NAMs to replace fish in vivo studies. Source: Draft position paper: Towards integration of NAMs supporting Environmental Safety ©CEFIC
Some of the key projects that are currently underway in ERASM in this area of research are:
- Research on eye damage and irritation work (EDI) to assess the reliability of existing in vitro assays for surfactants and their dilutions and develop and approach for setting SCLs based on in vitro data;
- Assessing the applicability of Physiologically-Based Kinetic (PBK) models for surfactants - with the objective to establish PBK as an in silico technique to estimate absorption, distribution and elimination;
- Assessing the potential of membrane-water partitioning (Kmw) as an alternative approach for understanding the hydrophobicity of surfactants for use in assessing in silico toxicity and bioaccumulation determination;
- Assessing the suitability of the RTgill-W1 cell line assay (OECD 249) for surfactants as an alternative to the fish acute toxicity test.

A.I.S.E. participates in the Informal Working Group on Non-Animal Testing Methods which is part of the UN Sub-Committee of Experts on the GHS.
The tenth revised edition of the GHS incorporated changes relating to the use of NATM for classification of health hazards, in particular skin corrosion/irritation (chapter 3.2), serious eye damage/eye irritation (chapter 3.3) and respiratory or skin sensitisation (chapter 3.4). These include, inter alia, classification based on in vitro/ex vivo test data and on the use of defined approaches (rule-based combinations of data obtained from a predefined set of information sources, e.g. in vitro methods, ex vivo methods, physico-chemical properties, non-test methods).
A.I.S.E also has new and ongoing projects on performance of in vitro methods for eye effects and alternative tests for eye and skin irritation. The publication can be downloaded below.
- Suitability of histopathology as an additional endpoint to the Isolated Chicken Eye Test for Classification of non-extreme pH Detergent and Cleaning Products. Toxicology In Vitro 28, 657-666. Cazelle et al, 2014.
- Suitability of the Isolated Chicken Eye Test for Classification of Extreme pH Detergents and Cleaning Products. Toxicology In Vitro 29, 609-616. Cazelle et al, 2015.
- Suitability of the Isolated Chicken Eye Test for Classification of Extreme pH Detergents and Cleaning Products- Revised version of OECD Test Guideline No. 438, 2018
- Inter-laboratory performance of ICE histopathology scoring to identify UN GHS Category 1 surfactants and non-extreme pH detergents. Regulatory Toxicology and Pharmacology Volume 126, November 2021, 105044. Eskes et al, 2021.

Collaboration with other downstream formulating sectors through the Downstream Users of Chemicals Coordination Group (DUCC) contributes, with a common voice, to the successful implementation of the requirements of the REACH and CLP Regulations across all sectors. This includes dialogue and coordination of activities with the European Commission and the European Chemicals Agency ECHA.
Related content: